Say goodbye to menopausal symptoms, positive results in the early stages of researching new drugs
June 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];KaNDy Therapeutics, a British biotechnology company, recently announced that its leading menopausal syndrome non-hormone drug candidate NT-814 has obtained positive data in the Phase 1b/2a clinical trial. The results suggest that NT-814, a novel, once-daily, oral neurokinin (NK) receptor-1,3 antagonist, can rapidly and comprehensively alleviate multiple debilitating symptoms of climacteric syndrome, including vasomotor Vasomotor symptoms (VMS), hot flashes/flushes (HF) and night awakening.

In the United States, 11 million women are naturally menopausal each year. The average age of menopause is 51.3 years old. More than 85% of women will feel uncomfortable due to insufficient estrogen. VMS occurs mostly in late menopause and early postmenopausal, or several years before and after the final menstrual cycle. 60-80% of women suffer from VMS more or less during the period of menopause. However, a series of recent studies have shown that a few early middle-aged women begin to have VMS symptoms as early as the menstrual cycle begins to change, and continue until decades after the menopause transition, as late as 60-70 years old. . Such diseases can plague women for many years and put tremendous pressure and financial burden on health care.
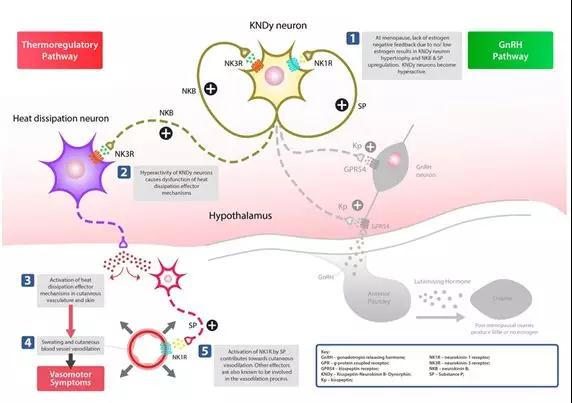
â–² NT-814 mechanism of action and corresponding pathway (Source: KaNDy official website)
KaNDy Therapeutics is committed to the development of NT-814, a common chronic disease drug associated with sex hormones. NT-814 is an oral, selective and highly potent small molecule NK-1,3 receptor bifunctional antagonist. There are three kinds of NK-1, 2, and 3 receptors in mammals, which can bind substance P, neurokinin A, and neurokinin B, respectively, non-specifically and selectively. Academics believe that NK-1, 2, 3 receptor antagonists can be used for anti-depression, anti-anxiety, anti-psychiatric disorders.
Scientific studies have shown that the NK-3 receptor system plays a mandatory regulatory role in the "KNDy" (kisspeptin-neurokinin B-dynorphin) neural network located in the hypothalamus, and there is evidence that it directly affects the cooling neurons of the hypothalamus. Thus, the NK-3 receptor system has a controlling effect on at least two major pathways: the sporadic thermoregulatory pathway, and the gonadotropin releasing hormone (GnRH) pathway. The latter is part of the hypothalamic-pituitary-gonadotropin (HPG) axis, which simultaneously regulates the release of sex steroid hormones. In addition, the NK-1 receptor system has also been shown to be expressed on human KNDy neurons and may also compensate for peripheral heat loss by promoting skin vasodilation (HFs). In fact, intravenous infusion of substance P through the arm can cause flushing of the face and neck, which is characteristic of VMS.
In menstruating women, estrogen produced by the ovaries has a feedback effect on KNDy neurons located in the hypothalamus, thereby regulating the reproductive cycle through the GnRH pathway/HPG axis. In postmenopausal women, the lack of estrogen and the resulting loss of modulation of KNDy neurons cause them to become large (hypertrophic) and overactive. It is understood that these events will destroy the normal function of the thermal effector mechanism, leading to debilitating symptoms of HF.
Thus, the dual NK-1,3 receptor antagonist NT-814 has the potential to reduce hyperactivity of the KNDy neural network in menopausal women by centrally inhibiting NK-3 receptor signaling on KNDy and heat-dissipative neurons. This will restore the heat dissipation effect to normality and solve the problem of dysregulation leading to HF symptoms. In addition, NT-814 blocking the NK-1 receptor around the blood vessels of the skin may also cause a decrease in the vasodilation response, thereby contributing to the reduction of HF intensity.
Phase 1b/2a study RELENT-1 is a randomized, double-blind, placebo-controlled study of three clinical pharmacology units (CPUs) in the United States. Sixty-seven women aged 41-64 years with moderate to severe HF were enrolled in the study and were randomized to receive a total of 14 days in 4 cohorts of 4 different doses of NT-814 or placebo. a treatment. Among them, the first 7 days reside in the CPU. Subjects completed diaries twice daily before and during treatment and performed safety assessments throughout the trial.
At the most effective dose evaluated, NT-814 was rapidly and significantly reduced:
At the frequency of HF, the moderate and severe HF frequency of HF decreased by 62% (p<0.0014) from baseline at week 1 and 24% for placebo; 84% (p<0.0002) at week 2, placebo 37%;
The severity of HF, the mean heart failure at week 1 was 23% lower than baseline (p < 0.015), 9% in the placebo group, 50% in the second week (p < 0.0004), and 16% in the placebo group. ;
The number of wake-ups at night was 58% lower than baseline at week 1 (p < 0.0022), 17% in placebo, 81% at week 2, and 32% at placebo (p < 0.0005).
NT-814 is well tolerated and has no safety issues throughout the dose range. Assessment methods include routine safety laboratories, liver function tests, electrocardiograms, and vital signs. Based on these results, KaNDy plans to conduct Phase 2b clinical trials later this year.

â–² Dr. Richard Anderson, Clinical Reproductive Science, University of Edinburgh (Source: KaNDy official website)
Dr. Richard Anderson, Ph.D., Clinical Reproductive Science, KaNDY Science and Clinical Consultant, University of Edinburgh, said: "These trials show that the new daily oral administration of NT-814 may be a treatment to replace HRT (hormone replacement therapy). It can help menopausal women to alleviate a series of debilitating symptoms. HRT may take weeks or even months to be fully effective. Compared with HRT, NT-814 not only has a rapid effect on relieving HF and multiple awakenings at night, but also has a curative effect. Significant."
We look forward to the positive results of the follow-up clinical phase 2 trials, providing more treatment options for menopausal women as soon as possible, and helping them to relieve menopausal symptoms as soon as possible!
Reference materials:
[1] KaNDy Therapeutics Announces Positive Clinical Data with Lead Candidate NT-814 for the Treatment of Symptoms of the Menopause
[2] Tachykinins and neuropsychiatric disorders
[3] The mammalian tachykinin receptors
[4] Vasomotor Symptoms and Menopause: Findings from the Study of Women's Health Across the Nation
[5] Managing Menopause
[6] KaNDY official website
China Extract Powder For Use As Dietary Supplement Extract Powder, Extract Powder Manufacturer
Shaanxi Kang New Pharmaceutical co., Ltd. , https://www.apipepdites.com