Summary of recent progress in the field of cancer (12.04)
December 04, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. The first Herceptin biosimilar drug was approved for the treatment of gastric cancer and breast cancer
Mylan and Biocon recently announced that the US FDA has approved the launch of Ogivri (trastuzumab-dkst) jointly developed by the two companies. Ogivri is a biosimilar of Herceptin (traffic name trastuzumab) approved for the treatment of all indications in Herceptin, including HER2 overexpressing and metastatic gastric cancer. (Glandular or esophageal stomach junction adenocarcinoma). This is also the first biosimilar to target these two specific cancers.

Ogivri is the first Herceptin biosimilar approved by the FDA. Biopharmaceuticals are usually derived from living organisms and can come from many sources such as humans, animals, microorganisms or yeast. A biosimilar is a biological product that, in addition to meeting other standards prescribed by law, is highly similar to the FDA-approved biologic (reference drug) and has no clinical significance in terms of safety, purity, and efficacy. The difference.
Ogivri's approval is based on extensive data review. This includes structural and functional characterization, animal research data, human pharmacokinetic and pharmacodynamic data, clinical immunogenicity data, and other clinical safety and efficacy data. The data shows that Ogivri is very similar to Herceptin. In July 2017, the FDA Oncology Drugs Advisory Committee (ODAC) voted unanimously to recommend the approval of Mylan's Herceptin biosimilar.
Ms. Heather Bresch, CEO of Mylan, commented: “Mylan's Ogivri approval provides patients with access to biosimilar drugs and also saves a lot of money for the US healthcare system. As a leading cancer drug supplier in the US, Mylan is very happy. Adding products to our portfolio that represent a new generation of targeted therapies that have revolutionized the way cancer is treated. Ogivri is one of the many biosimilars in our pipeline, as our commitment to continue to deliver important drugs to patients a part of."

Ms. Kiran Mazumdar-Shaw, Chairman and Managing Director of Biocon, said: “The FDA has approved our trastuzumab biosimilars, which is the culmination of a global biosimilar drug that strengthens our commitment to development at a reasonable price. The determination of biologics to make cancer recovery more effective and fairer worldwide is an important milestone in our development of advanced therapies that are expected to benefit billions of patients."
Mylan and Biocon's Herceptin biosimilars are being reviewed by regulators in Australia, Canada, the European Union and other markets. It has been approved in 19 countries around the world to provide cancer patients with more affordable treatment options. We expect this new drug to enable more cancer patients to use affordable drugs to recover from cancer as soon as possible.
2. Total survival benefits are significant, Opidivo is expected to enter China in the near future
Bristol-Myers Squibb (NYSE: BMY) announced that it has been based on a Phase III clinical trial of the efficacy of nivolumab versus docetaxel in patients with advanced or metastatic non-small cell lung cancer (NSCLC). CheckMate-078 As a result, the Independent Data Surveillance Committee (DMC) determined that the study had reached its primary endpoint, ie, a significant overall survival (OS) benefit was observed in patients receiving nivolumab compared to the control group, so the study was advanced End. The safety of nivolumab is consistent with the previously reported data on solid tumor studies. CheckMate-078 is a multi-country, phase III clinical study based on Chinese patients. At present, Bristol-Myers Squibb has submitted a listing application (BLA) of nivolumab to the China National Food and Drug Administration (CFDA), and its target indication is treated non-small cell lung cancer (NSCLC). CFDA has accepted the application for listing of nivolumab.

Professor Wu Yilong, the lifelong director of the Guangdong Provincial Lung Cancer Institute and the Guangdong Provincial People's Hospital, the chairman of the China Thoracic Oncology Research Collaborative Group (CTONG) and the leading investigator of CheckMate-078, said: "This time, the CheckMate-078 Phase III clinical trial reached the end point early. The encouraging results, for the first time, confirmed that the PD-1 immune checkpoint inhibitor nivolumab has a significant overall survival benefit compared to the standard treatment of docetaxel in the Chinese population. Based on the initial results of this study, nivolumab is expected To become the first immunotherapy (IO) treatment option for Chinese patients with lung cancer, I hope that patients with non-small cell lung cancer (NSCLC) in China will benefit from this epoch-making immune tumor (IO) treatment."
3. The first "breakthrough" cancer in vitro diagnostic test was approved
Recently, the US FDA announced the approval of FoundationOne CDx (F1CDx) of WuXi PharmaTech Group Partner Medicine. This is the first breakthrough-based next-generation sequencing (NGS) in vitro diagnostic (IVD) test that can diagnose any solid tumor, find genetic mutations within 324 genes, and two types of genomic features.

This is a big step forward in precision medicine. Previously, we have learned to “prescribe the right medicine†for patients with specific mutations, and the detection of these mutations is often accompanied by a concomitant diagnosis approved at the same stage as the new drug. Although such tests are accurate, they can only serve one-on-one for a specific new drug.
The use of F1CDx is more extensive. It diagnoses many different mutations and provides critical information to help cancer patients manage their disease. In addition, in patients with non-small cell lung cancer, melanoma, breast cancer, colorectal cancer, and ovarian cancer, it can also identify which patients can benefit from the 15 targeted therapies approved by the FDA. What the patient and the doctor have to do is just a simple test, no need to repeat the biopsy.

In the test, F1CDx was able to detect gene mutations (base substitutions, or short insertions/deletions) in patient solid tumors, as well as microsatellite instability and tumor mutation burden. In clinical performance, this test was compared to a previous FDA-approved companion diagnosis. The researchers found that the F1CDx data was close to 94.6% in terms of overall accuracy.
"Using two major accelerated innovation technologies, we are able to bring breakthrough diagnostic methods to patients faster, help doctors decide on cancer treatment options, improve prognosis, and reduce medical costs," said FDA Director Dr. Scott Gottlieb.
“F1CDx can help cancer patients and their doctors get more information to make medical decisions without frequent invasive testing. Previous tests require tumor samples to be extracted many times before deciding whether to take monotherapy or participate. In clinical trials, Dr. Jeffrey Shuren of the FDA Medical Devices and Radiation Health Center (CDRH) said: "With a single test, patients and doctors can evaluate multiple disease management programs."
4. For CCR4 , new lymphoma drugs are eligible for priority review
Recently, Kyowa Hakko Kirin announced that the US FDA has accepted the mogamulizumab Biologics Licensing Application (BLA) for the treatment of patients with cutaneous T-cell lymphoma (CTCL) who have received at least one systemic treatment and has been granted priority review. .

CTCL is a rare non-Hodgkin's T-cell lymphoma. The two most common types of CTCL are mycosis fungoides (MF) and Sezary syndrome (SS). According to the stage, the disease may involve skin, blood, lymph nodes and internal organs. Late stage CTCL is associated with significant morbidity and mortality. These patients are in desperate need of new treatments to alleviate the disease.
Kyowa Hakko's mogamulizumab, developed using its proprietary POTELLIGENT® platform, is a humanized monoclonal antibody (mAb) targeting CC chemokine receptor 4 (CCR4), which is frequently found in certain hematologic malignancies (including CTCL). Expressed on leukemia cells. Mogamulizumab was first approved for the treatment of other malignant hematologic tumors in Japan in 2012 and was approved for the treatment of CTCL in 2014. The US FDA has awarded mogamulizumab a breakthrough therapy for the treatment of MF and SS patients who have received at least one systemic treatment. The mogamulizumab was once again eligible for priority review, proving its potential in treating CTCL.
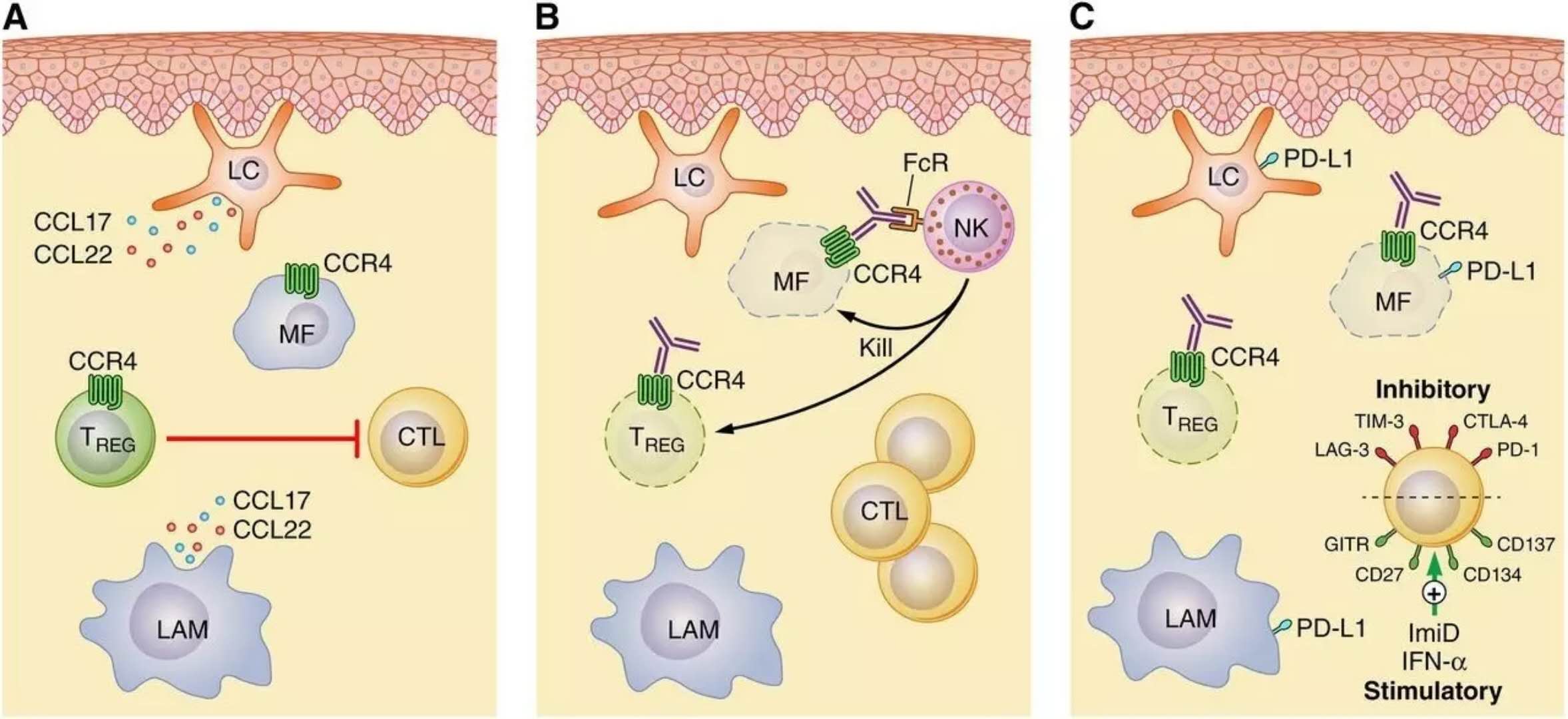
â–²CCR4 is a therapeutic target for cutaneous T-cell lymphoma (Source: Blood Journal)
The efficacy of Mogamulizumab was validated in a phase 3 clinical trial MAVORIC. This open-label, multicenter, randomized trial evaluated the efficacy of mogamulizumab in patients with MF and SS who had failed after at least one systemic treatment. It recruited 372 patients in the United States, Europe, Japan, and Australia. The top line results demonstrated a significant improvement in progression-free survival (PFS) in patients treated with mogamulizumab compared to the control group, while being well tolerated.
“I am very pleased that the FDA has accepted the BLA of mogamulizumab and granted priority review, which is another major achievement of our subsidiary Kyowa Kirin Pharmaceutical Development,†said Dr. Mitsuo Satoh, Vice President and Executive Director of Kyowa Hakko Kirin. We will continue to work with regulatory agencies such as the FDA to provide this drug to CTCL patients in the United States as soon as possible."
5. CDK7 inhibitor new drug begins clinical phase 1 trial
Carrick Therapeutics has announced that the Phase 1 clinical trial to evaluate the safety and efficacy of CT7001 has officially begun, and the first patient to participate in the study has begun to receive medication. CT7001 is an oral cyclin-dependent kinase 7 (CDK7) inhibitor that has demonstrated superior efficacy in a variety of preclinically studied cancer models.

In recent years, inhibition of CDK7 has become a potential therapeutic strategy in a variety of cancers. CDK7 is a major regulator of transcription and regulates the cell cycle by phosphorylating CDK family members. Inhibition of CDK7 inhibits the expression of key oncogenes such as c-Myc. Data from preclinical studies show that CT7001, which inhibits CDK7, has good anticancer effects in hormone receptor-positive and triple-negative breast cancer, as well as in transcription factor-driven cancers such as acute myeloid leukemia (AML) and small cell lung cancer (SCLC). effect. These cancers still have significant unmet medical needs. And, because of their different mechanisms of action, developers believe that CT7001 is also effective against cancers that are already resistant to current treatments.
“In less than two years, pushing preclinical candidates to the stage where clinical trials can begin in patients is a major achievement for Carrick Therapeutics,†said Elaine Sullivan, CEO of the company: “We I am excited about the huge potential of CT7001 in cancer treatment, and I intend to rapidly advance the development of CT7001 through clinical trials and provide patients with such promising new drugs as soon as possible."
Reference materials:
[1] FDA approves first biosimilar for the treatment of certain breast and stomach cancers
[2] US FDA Approves Mylan and Biocon's OgivriTM, the First Biosimilar for Trastuzumab, for the Treatment of HER2-Positive Breast and Gastric Cancers
[3] FDA announces approval, CMS proposes coverage of first breakthrough-designated test to detect extensive number of cancer biomarkers
[4]Kyowa Hakko Kirin Announces FDA Acceptance for Filing and Priority Review Designation of Mogamulizumab's Biologics License Application
[5] Carrick Therapeutics Announces First Patient Dosed in Phase I Clinical Trial of its Oral CDK7 Inhibitor: CT7001
Original title: Summary of recent progress in the field of cancer (No. 47)
Medical Hospital Bed,Patient Bed,Electric Hospital Bed,Adjustable Hospital Bed
Hebei Dingli Medical Equipment Co., Ltd. , https://www.dinglimed.com