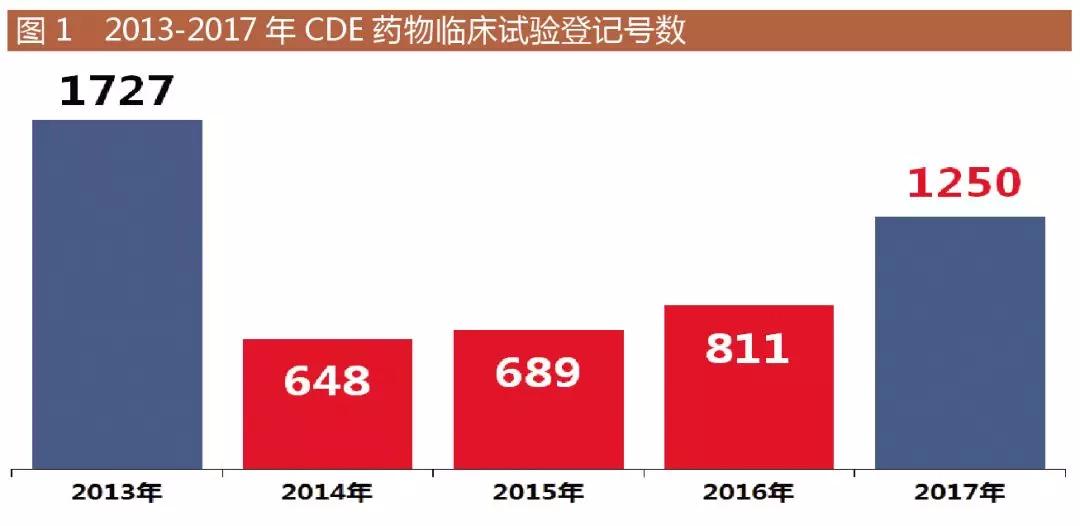
Medical Network January 9th As of January 3, 2018, 2017 CDE drug clinical trial registration and information disclosure platform data returned to four digits, as shown in Figure 1, rebounded to 1,250 registration numbers, is near A four-year high. From the indications, tumors and cardiovascular and cerebrovascular related drugs are still hot spots, and drugs for preventing thrombosis are gradually increasing.
The 2017 compliance evaluation policy was released, and generic drugs recovered. The comments on the approval of priority review for drug innovations issued at the end of 2017 were clear, and generic drugs with expired patents could be reviewed first. So, what are the projects that companies must strive for and rank quality?
The author further analyzes the clinical project initiated in 2017, and sorts out the list of products that the patent expired generic drugs are to be included in the priority review, and explores the new pattern of domestic R&D competition in 2018.
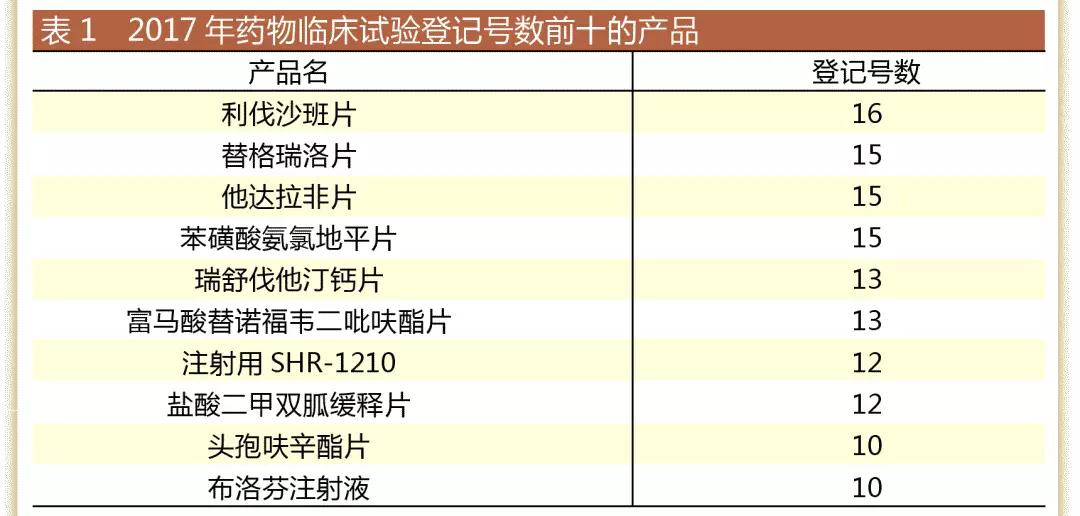
Clinical registration number TOP 10 :
Generic fever returns
As shown in Table 1, 9 of the top 10 products in the registration number are generic drugs, and the top five are all generic drugs. It can be seen that the clinical projects initiated in 2017 are mainly generic drugs.
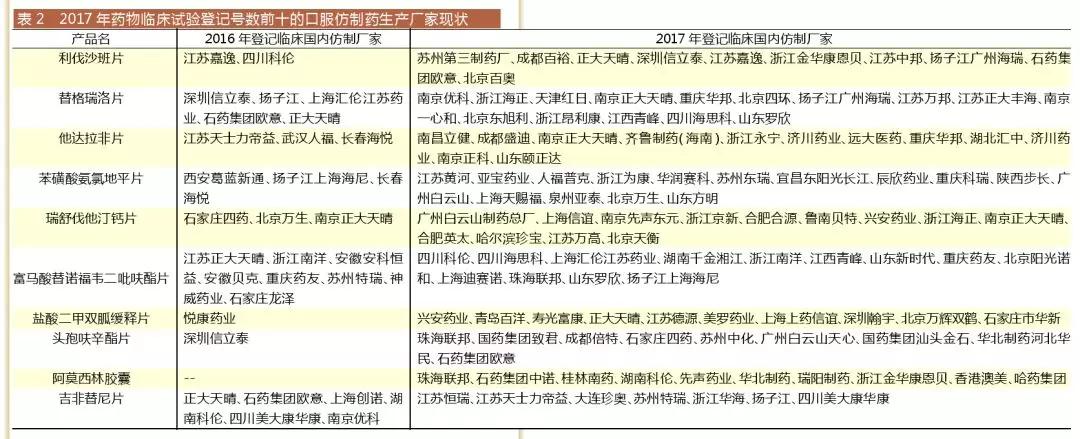
Salta Data V3.5 compared to the 2017 drug clinical trial registration number of the top ten oral generic drugs corresponding to the 2016 and 2017 domestic imitation manufacturers found, in addition to tenofovir fumarate tablets and Jifei After the imitation of the first generic drug manufacturer in 2016, the number of generic drug manufacturers registered in 2017 was much higher than that of 2016.

Report hotspots:
Cardiovascular drugs, anticoagulants, 289 varieties
In 2017, the number of clinical registrations of generic drugs reached a new high. People can't help but recall that before the start of the clinical self-inspection and verification in 2015, major R&D and production companies were paying close attention to the approval order of the first generic drug clinical approvals. The higher the market value of the acceptance number.
After the 2015 start clinical checking and verification, conformance assessment before the relevant policy documents published clinical batch release this document, the forefront of the rankings no longer the only measure of the market value, the importance of improving the quality of the project. During this period, major generic drug R&D and production enterprises entered a period of silence, and the hottest application of innovative drugs was not reduced. The “double-reported†products may be listed on the domestic market and the policy of obtaining a consistent evaluation title will promote the “going outâ€.
In 2017, the relevant policies for the consistency evaluation were released, and the generic drugs recovered. Enterprises must strive for ranking and strive for the quality of the project to pass smoothly.
From the point of view of indications, cardio-cerebral-related, anti-thrombosis and anti-coagulation generic drugs are hot spots. In addition, the competitive situation of products with larger sales in the 289 catalogue is also fierce.
Patent-expired drugs are included in the priority review:
Product List
On December 28, 2017, the State Food and Drug Administration issued an opinion on encouraging priority review and approval of drug innovation. Accordingly, the two types of generic drugs received a priority review – three years prior to the expiration of the drug clinical trial application and one year prior to the expiration of the drug production application; and the same production line produced in China and in the United States, EU drugs The examination and approval authority will apply for registration of drugs that are simultaneously applied for listing and passed the on-site inspection.
As of January 3, 2018, a total of 41 acceptance numbers entered the list of products that the CDE intends to include in the priority review process for drug registration due to the expiration of the patent. The “first batch of priority review patent expiration varieties and applicants list†corresponds to 14 Only one of the acceptance numbers is a clinical application. There is only one acceptance number for the clinical application three years before the patent expires, and there are 26 acceptance numbers for the pharmaceutical production application one year before the patent expires.

From the manufacturer's point of view, the products of Zhejiang Haizheng, Zhengda Tianqing, Jiangsu Hengrui and Jiangsu Haosen entered the number of acceptances for the drug registration application to be included in the priority review procedure.
Outlook<<<
The first batch of conformity evaluation has been announced, which will further stimulate R & D institutions and production companies to register clinical progress in 2018. Due to the mature market cultivation of generic drugs, R&D institutions and manufacturers will seize the title of consistency evaluation of generic drugs under the promotion of consistency evaluation and MAH system, and it is expected to break the existing competition pattern in the approval of the reshuffle. It is expected that the new pattern in 2018 will be the battle for the market's larger products and the patent-based consistency evaluation of patents.
Xi'an Healthway Biotech Co.,Ltd , https://www.xianhealthway.com