How to Identify and Prevent Jujube Anthracnose
Model NO.: PI108
Pharmaceutical Technology: Chemical Synthesis
Drug Reg./Approval No.: S50312
Specification: 20%20ml
Origin: China
Model NO.: PI108
Pharmaceutical Technology: Chemical Synthesis
Drug Reg./Approval No.: S50312
Specification: 20%20ml
Origin: China
Function of the attending
It can be used as anticonvulsant.
Often used in pregnancy hypertension.
Lowering blood pressure, treating preeclampsia and preeclampsia, also used to treat premature birth.
Â
contraindication
It's not clear.
Â
Usage.
1. Treatment of moderately severe gestational hypertension, preeclampsia and eclampsia dose was 2.5 ~ 4 g, for the first time in 25% glucose injection diluted with 20 ml 5 minutes slow intravenous injection, 1 ~ 2 g per hour after an intravenous drip.
The total amount of 24 hours was 30g, according to the knee tendon reflex, the number of breaths and the amount of urine monitoring.
2. The dosage and method of treatment for preterm labor and treatment of pregnancy hypertension were similar, and the initial load was 4g.
With 25% grape pond within 5 minutes after injection diluted 20 ml slow intravenous injection, after using 25% magnesium sulfate injection 60 ml, 5% on grape pond injection intravenous drip in 1000 ml, the rate of 2 g per hour, until contractions stop after 2 hours, to maintain oral beta-adrenergic receptors excited after medicine.
3. Treatment of convulsion or intravenous medication in children: 0.1 ~ 0.15g/kg each time, with 5%~10% glucose injection diluted into 1% solution, intravenous drip or diluted into 5% solution, slow static injection.
A 25% solution can be used as a deep intramuscular injection.
In general, pediatrics is safe only by intravenous or intravenous administration.
Â
Matters needing attention
1. Before applying magnesium sulfate injection, the function of kidney function should be examined. If renal function is not fully used, the dosage should be reduced.
2. Should be used with caution or not in case of myocardial damage and cardiac arrest.
3. Before each use and use process, the timing for a knee tendon reflex test, determination of breathing, to observe the urine output, exsanguinate check blood magnesium concentrations as a knee tendon reflex significantly weakened or destroyed, or breathing number less than 14 ~ 16 times a minute, an hour less than 25 ~ 30 ml of urine 24 hours or less than 600 ml, should be stopped in time.
4. Sudden chest tightness, chest pain and shortness of breath in the course of the medication should be listened to timely, and the chest X-ray film should be needed to detect the pulmonary edema as early as possible.
5. If there is an acute magnesium poisoning, calcium injection can be used to relieve the calcium injection, and 10ml of calcium lepate injection is commonly used.
6. When treatment is carried out, it is not appropriate to use the adrenalin beta receptor agonist, such as ritodrine, which can cause adverse cardiovascular reactions.
Â
Adverse reaction
1. Intravenous magnesium sulfate often cause symptoms such as hot flashes, sweating, dry mouth, when rapid intravenous injection can cause nausea, vomiting, flustered, giddy, individual nystagmus, slow down the injection speed symptoms can disappear.
2. Renal insufficiency, large dosage, can happen blood magnesium accumulation, blood magnesium concentration up to 5 tendency/L, can appear the muscle excitability inhibition, feel sluggish, knee tendon reflex disappearing, is suppressed in the breath, the blood concentration of magnesium tendency for up to 6 L can occur when breathing stops and arrhythmia, heart block, higher concentration further, can make the heart stopped.
3. Continuous use of magnesium sulfate can cause constipation, and some patients can have paralytic ileus and improve after stopping.
4. Very few blood calcium decreases, reappearance hypocalcemia.
5. Magnesium ion can be free through the placenta, resulting in neonatal high blood magnesium syndrome, which is characterized by low muscular tension, poor sucking ability, inactivity, poor crying, etc., and a few respiratory depression.
6. A few pregnant women have pulmonary edema.
Â
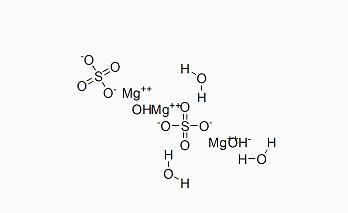
The main ingredient of this product is magnesium sulfate
Â
Dosage form
injection
Â
Drug interaction
With magnesium sulfate compatibility taboo polymyxin B, streptomycin sulphate, grape pond acid calcium, dobutamine hydrochloride, procaine hydrochloride, tetracycline, penicillin and naphthalene Westwood oxygen naphthalene penicillin (B).
Pharmacological action
Magnesium ions can inhibit the activity of central nervous, restrain the nerve - muscle joint acetylcholine release, blocking transmission of nerve muscle connection place, reduce or remove muscle contraction function, at the same time of vascular smooth muscle relaxation effect, make the convulsion of peripheral vascular expansion, lower blood pressure, and the prevention and treatment for pre-eclampsia, also have inhibition to uterine smooth muscle contraction, and can be used in the treatment of premature birth.
Â
Storage method
Save in a closed shade.
Â

Function of the attending
It can be used as anticonvulsant.
Often used in pregnancy hypertension.
Lowering blood pressure, treating preeclampsia and preeclampsia, also used to treat premature birth.
Â
contraindication
It's not clear.
Â
Usage.
1. Treatment of moderately severe gestational hypertension, preeclampsia and eclampsia dose was 2.5 ~ 4 g, for the first time in 25% glucose injection diluted with 20 ml 5 minutes slow intravenous injection, 1 ~ 2 g per hour after an intravenous drip.
The total amount of 24 hours was 30g, according to the knee tendon reflex, the number of breaths and the amount of urine monitoring.
2. The dosage and method of treatment for preterm labor and treatment of pregnancy hypertension were similar, and the initial load was 4g.
With 25% grape pond within 5 minutes after injection diluted 20 ml slow intravenous injection, after using 25% magnesium sulfate injection 60 ml, 5% on grape pond injection intravenous drip in 1000 ml, the rate of 2 g per hour, until contractions stop after 2 hours, to maintain oral beta-adrenergic receptors excited after medicine.
3. Treatment of convulsion or intravenous medication in children: 0.1 ~ 0.15g/kg each time, with 5%~10% glucose injection diluted into 1% solution, intravenous drip or diluted into 5% solution, slow static injection.
A 25% solution can be used as a deep intramuscular injection.
In general, pediatrics is safe only by intravenous or intravenous administration.
Â
Matters needing attention
1. Before applying magnesium sulfate injection, the function of kidney function should be examined. If renal function is not fully used, the dosage should be reduced.
2. Should be used with caution or not in case of myocardial damage and cardiac arrest.
3. Before each use and use process, the timing for a knee tendon reflex test, determination of breathing, to observe the urine output, exsanguinate check blood magnesium concentrations as a knee tendon reflex significantly weakened or destroyed, or breathing number less than 14 ~ 16 times a minute, an hour less than 25 ~ 30 ml of urine 24 hours or less than 600 ml, should be stopped in time.
4. Sudden chest tightness, chest pain and shortness of breath in the course of the medication should be listened to timely, and the chest X-ray film should be needed to detect the pulmonary edema as early as possible.
5. If there is an acute magnesium poisoning, calcium injection can be used to relieve the calcium injection, and 10ml of calcium lepate injection is commonly used.
6. When treatment is carried out, it is not appropriate to use the adrenalin beta receptor agonist, such as ritodrine, which can cause adverse cardiovascular reactions.
Â
Adverse reaction
1. Intravenous magnesium sulfate often cause symptoms such as hot flashes, sweating, dry mouth, when rapid intravenous injection can cause nausea, vomiting, flustered, giddy, individual nystagmus, slow down the injection speed symptoms can disappear.
2. Renal insufficiency, large dosage, can happen blood magnesium accumulation, blood magnesium concentration up to 5 tendency/L, can appear the muscle excitability inhibition, feel sluggish, knee tendon reflex disappearing, is suppressed in the breath, the blood concentration of magnesium tendency for up to 6 L can occur when breathing stops and arrhythmia, heart block, higher concentration further, can make the heart stopped.
3. Continuous use of magnesium sulfate can cause constipation, and some patients can have paralytic ileus and improve after stopping.
4. Very few blood calcium decreases, reappearance hypocalcemia.
5. Magnesium ion can be free through the placenta, resulting in neonatal high blood magnesium syndrome, which is characterized by low muscular tension, poor sucking ability, inactivity, poor crying, etc., and a few respiratory depression.
6. A few pregnant women have pulmonary edema.
Â
The main ingredient of this product is magnesium sulfate
Â
Dosage form
injection
Â
Drug interaction
With magnesium sulfate compatibility taboo polymyxin B, streptomycin sulphate, grape pond acid calcium, dobutamine hydrochloride, procaine hydrochloride, tetracycline, penicillin and naphthalene Westwood oxygen naphthalene penicillin (B).
Pharmacological action
Magnesium ions can inhibit the activity of central nervous, restrain the nerve - muscle joint acetylcholine release, blocking transmission of nerve muscle connection place, reduce or remove muscle contraction function, at the same time of vascular smooth muscle relaxation effect, make the convulsion of peripheral vascular expansion, lower blood pressure, and the prevention and treatment for pre-eclampsia, also have inhibition to uterine smooth muscle contraction, and can be used in the treatment of premature birth.
Â
Storage method
Save in a closed shade.
Â
Rotary Tattoo Machine,Stigma Tattoo Machine,Bizarre Tattoo Machine
Beyond E-Commerce Firm , http://www.ywtattooneedle.com