US federal health officials are calling for defibrillator manufacturers to solve the long-standing problems of such emergency medical devices. These problems have caused dozens of recalls in recent years and caused several casualties.
On November 15th, the US Food and Drug Administration (FDA) stated that for many years, external defibrillators have suffered from design and manufacturing deficiencies, and occasionally they have been unable to work properly in life and death.
In 2009, the FDA issued 17 recall announcements for cardiac defibrillators, which was a significant increase over 9 times in 2005. More than 28,000 issues related to defibrillators have been reported to the FDA over the past five years.
When the patient is in danger of sudden cardiac arrest, the defibrillator uses shocks to return his heart to normal. External defibrillators were once considered a high-tech device for the emergency room. Nowadays such devices are found in airports, office buildings, stadiums, and schools.
However, the FDA said that companies manufacturing defibrillators (including Philips Healthcare, American Heart Science, and others) have not solved the related problems that have led to the recall of thousands of devices.
Jeffrey Shuren, director of the FDA's Center for Medical Devices, said: “We have seen that the prevalence of external defibrillator safety issues is unusual and it requires a comprehensive multi-pronged approach. Many of the issues we find are preventable and Correctively, they may affect patient safety."
FDA listed issues include: faulty circuits that can cause defibrillators to fail; cluttered designs that make the defibrillator difficult to use; and production standards that cause defibrillator defects.
Spokespersons for Philips Healthcare and American Heart Science did not comment on the matter. Other manufacturers of external defibrillators also include Defibtech and Welch Allyn.
According to FDA officials, manufacturers usually solve problems one by one, rather than solving the larger quality problems of the devices they produce.
For example, a company has tracked hundreds of reports that all have a common flaw in the defibrillators they produce. The company solved this problem for each device one by one, but it never issued an announcement to remind the owner of the defibrillator to pay attention to this issue.
The FDA also stated that it has found a problem with the circuit that causes the defibrillator to automatically shut down, which may have caused the death of several patients.
On November 15, the FDA sent a letter to all defibrillator manufacturers asking them to hold talks with the government to discuss the repair and improvement of defibrillators. According to the plan, the FDA will hold a public meeting with government, industry, and academic scientists on this issue next month.
The FDA is also reviewing its approval procedures for new defibrillators. Currently, if the new defibrillators are similar to those defibrillators already on the market, they can be approved by accelerating the approval process without having to accept new research work.
External defibrillators usually include two plastic pads attached to the patient's chest to detect whether the patient's heart has abnormal heart rhythm. If the problem can be corrected (approximately a quarter of the time it is), then the plastic pad will give a shock and return the heart to normal.
According to FDA data, nearly 300,000 people in the United States are dying each year because of cardiac arrest.
Dr. John Weisfeldt of Johns Hopkins Hospital estimates that because of the use of external defibrillators by patients, 500 lives are saved each year in the United States. In the past five years, about 1 million external defibrillators have been sold.
Ralph Sacco, president of the American Heart Association (AHA) and the University of Miami, said research has shown that external defibrillators have saved the lives of millions of victims of sudden cardiac arrest. “We deeply feel that stoppages can cause sudden cardiac arrest. In the most common abnormal heart rhythm, external defibrillators are the only effective treatment."
Dr. Douglas Zipes, the former chairman of the American College of Cardiology (ACC), helped inventing the implanted defibrillator. He said that he was convinced of the value of an external defibrillator. For this reason, a few years ago, he would The external defibrillator was installed at home in Indianapolis.
Zipes said: "It's like an oversized mailbox installed on my lawn."
Iris Iriscope Iridology Camera:
Iris holographic diagnostics is a kind of knowledge that applies non-drug and natural therapy to analyze and care for the body by analyzing the iris and pupil to analyze and regulate the health condition of the body. In the field of health medicine, it is called non-invasive health testing and non-drug health intervention. It is a traditional clinical health medicine in Europe.
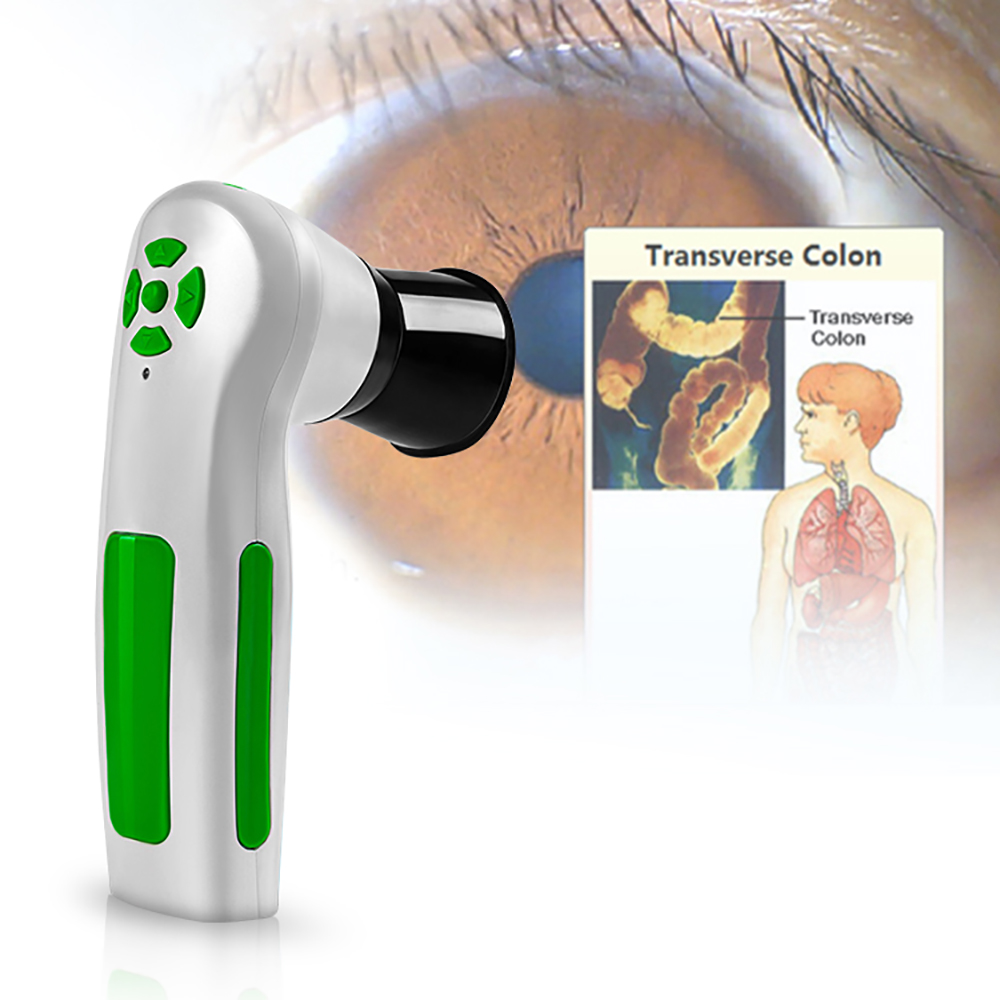
Iris Iriscope Iridology Camera System Functions:
1. Adjust brightness through either software or switch on handle line, delete the photos, and adjust the focus, quick fixed photos with white balance and high stability of colors. Connects directly with computer without outside power and easy to operate.
2. The software can save the client information and irises also the details of products that you recommend them. After taken the photos, you can analysis the irises and later compare the irises pictures when your client comes back to see their progress. Can print an analysis report. Save the photos according to the date & time you take the photos.3. This machine will help the client know his health condition, including the problem which you have had in the past. The Iridology will be your health counselor, tell you how to keep away from the illness.

Iris Iriscope Iridology Camera Specification:
* Nice appearance and innovative design
* LED illuminator around lens
* Imported lens with plated layer
* 12MP high resolution CCD sensor
* Special DSP image processor, Optical Image Stabilizer
* Single capture button and digital pause capture.
* Adjustable focus to give clear image.
* Auto white balance and contrast adjustment, Color Temperature Filter
* Compatible with iris lens, hair lens.
* Deliver clear and accurate images.
* Easy to operate.
* OS: Windows XP, WIN2000, 2003, Vista, Win7. Win8,win 10
What is the primary benefit of photo iridology?
Not only does it bring clues to your iridology analysis, but it helps to create a better connection with your patient. The patient, who`s naturally curious, is eager to see their own iris. With some simple explanations they can get a basic understanding of your analysis. The connection with your patient strengthens and their understanding of their process improves.
* Iris analysis system: international technology, unique functions.
* Iris analysis system is a medicinal tool that checks the body conditions and prevents diseases from occurring.
* We brought in the advanced iris analysis technology from Germany to lead people to discover sources of illness, and care the body health and spirit in anyways.
* The instrument can show the body conditions of customers and suggest customers the suitable health food, and the plans to care their bodies.
Iridology Camera Health Analyzer
Iridology Camera,Usb Iridology Camera,Iriscope Iridology Camera,Iridology Camera Eye Iriscope
Shenzhen Guangyang Zhongkang Technology Co., Ltd , https://www.lighttherapymachine.com